AI-powered Quality Oversight & Site Risk Management
Predict, manage, and mitigate site risk globally with AI-driven compliance.
Measurable Impact for Life Sciences Leaders
Predictive Risk Insights
Spot site compliance issues early and prevent costly disruptions.
65% Faster Qualification
Access 5.000+ ready-to-use audits with 450+ new reports monthly.
40% Lower Audit Costs
Switch to flexible, on-demand audits with our global network of expert auditors.
Audits that protect, Insights that prevent. AI-powered. Proactive. Outcome-driven.
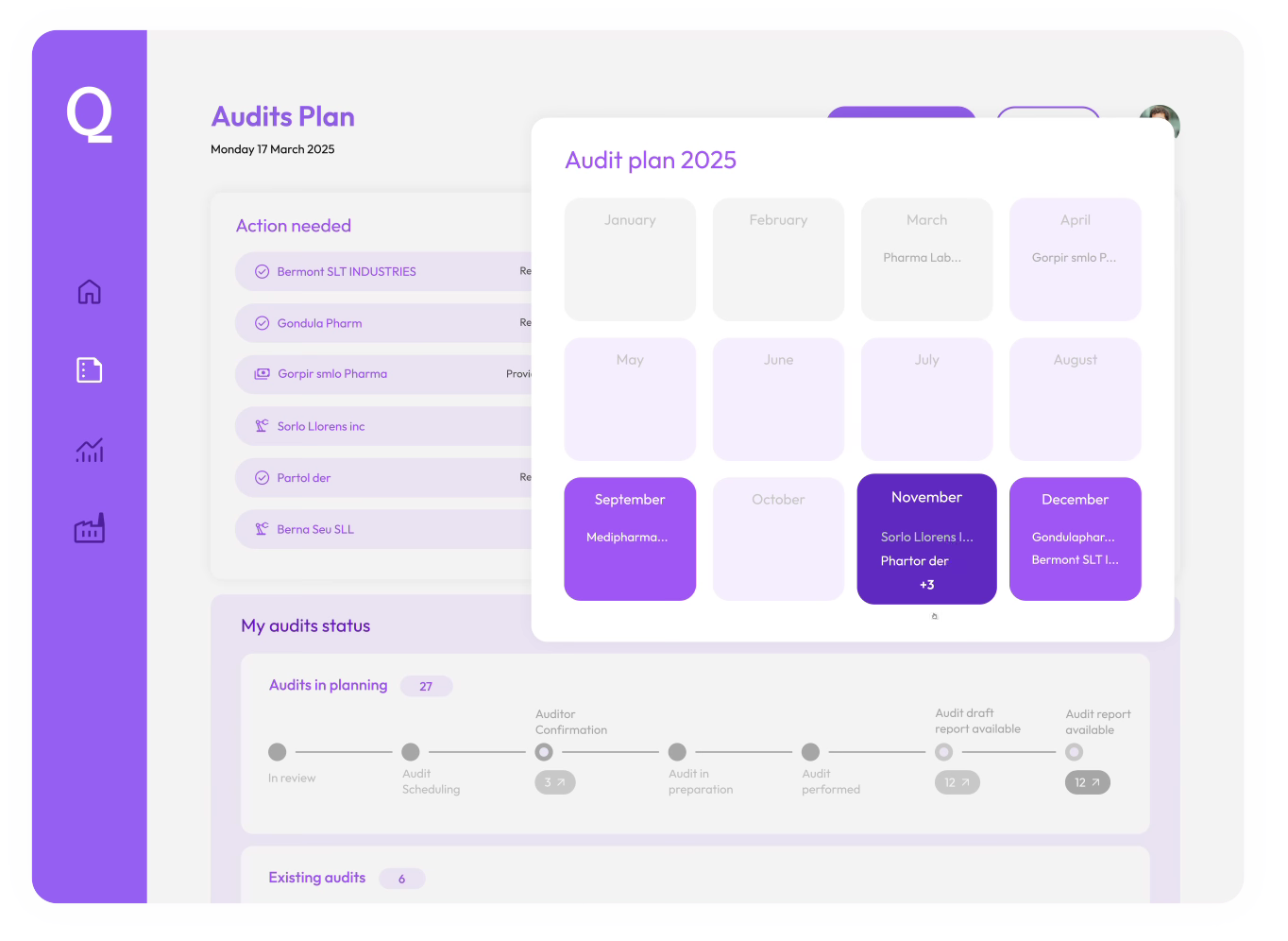
Audit Platform
Streamline audit execution and manage compliance at scale with ease
Real-time audit visibility
Monitor upcoming audits, track progress, and quickly access reports, all in one intuitive platform.
Optimized audit planning
Identify follow-ups, check renewal audit availability, and request new audits seamlessly.
Accelerated supplier qualification
Instantly access our extensive library of 5.000+ audit reports, reducing audit execution time by 65%.
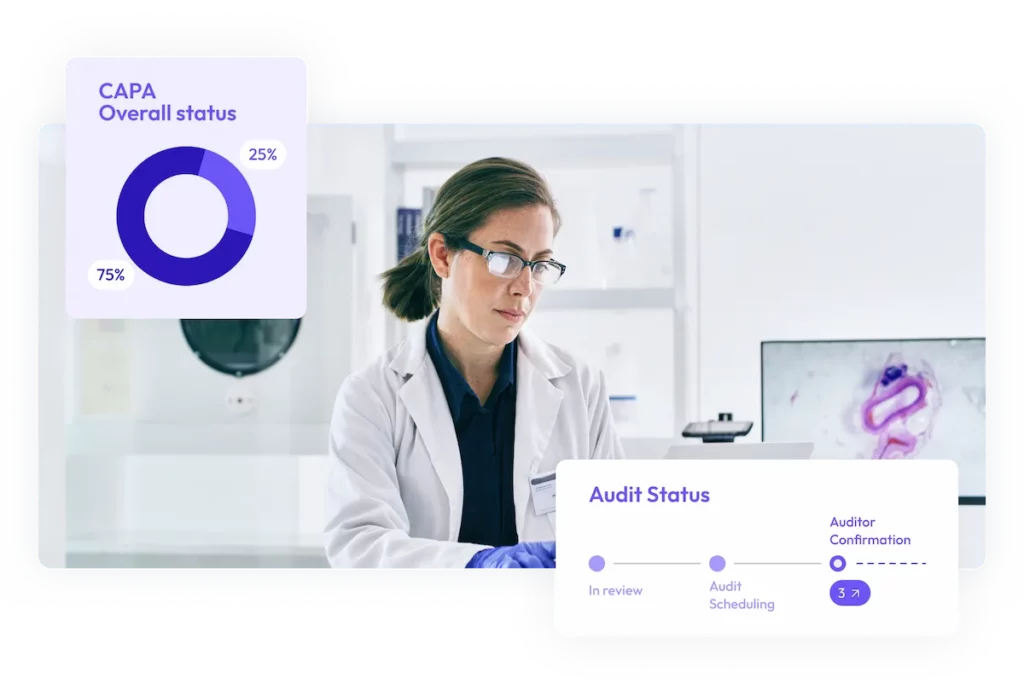
Digital CAPA management
Efficiently manage and close CAPAs with automated tracking, actionable alerts, and clear compliance dashboards.
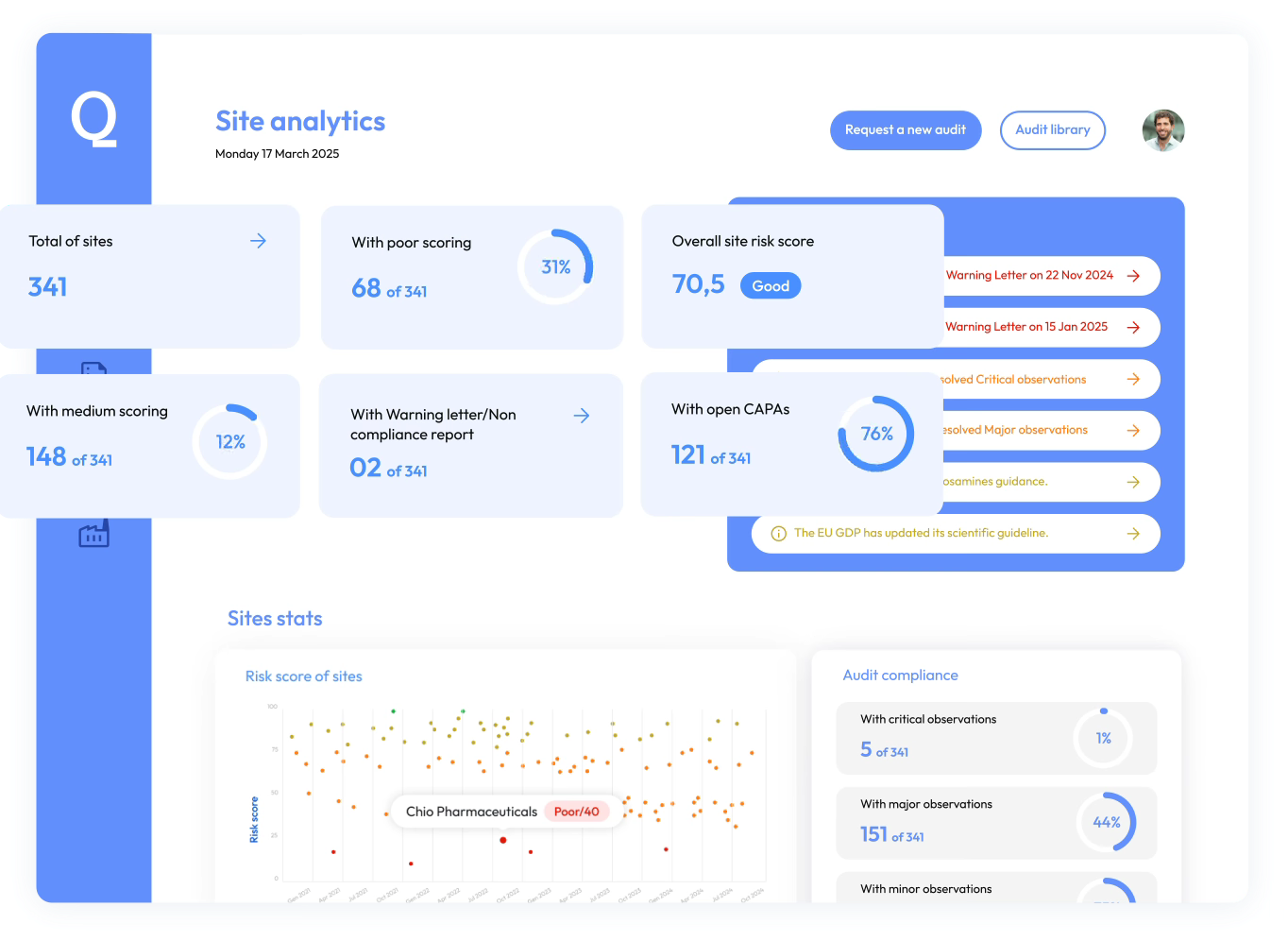
Quality Insights Platform
Predict and manage Site Risks Proactively with AI-Driven Insights.
Proactive site risk management
Identify issues before they become critical, minimize disruptions, and maintain a resilient supply chain.
Smarter, data-driven procurement
Select the best suppliers faster, avoid last-minute surprises, and secure reliable alternatives when needed.
Seamless collaboration across teams
Break down information silos with shared, standardized data, enabling faster decision-making and operational efficiency.
Tailored solutions built for your needs
Own supplier quality data
Digitize supplier site data, simplify compliance, and proactively manage risks.
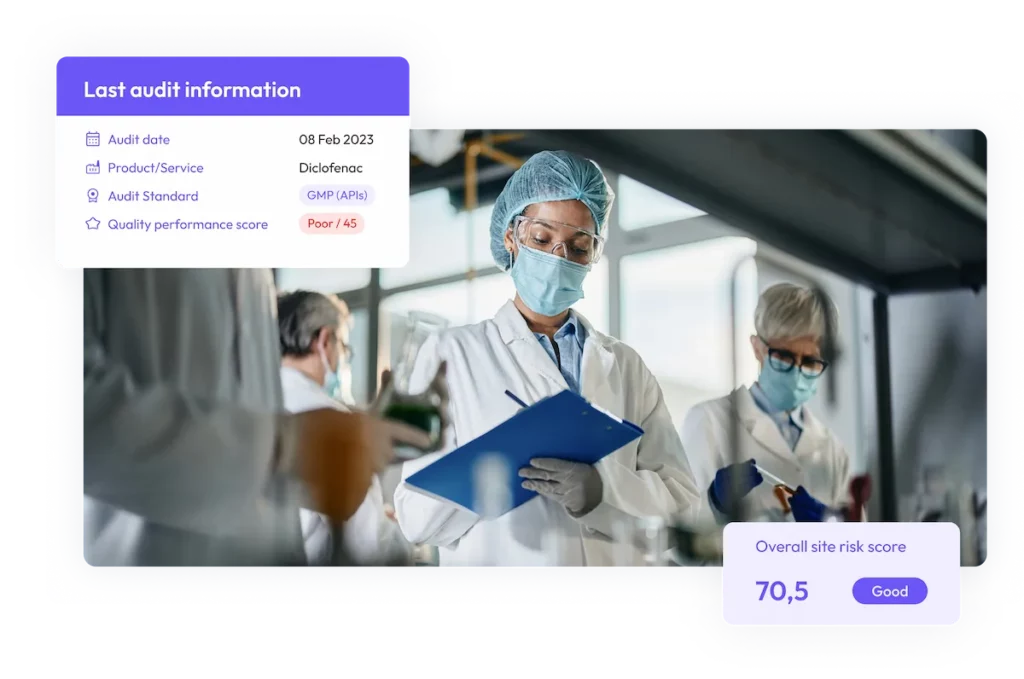
Track audits in real time
Streamline audits, monitor CAPAs, and access site quality risk assessments in ensure compliance visibility in real-time.
Qualify suppliers, fast
Accelerate supplier selection, minimize sourcing risks, and secure trusted suppliers faster.

Trusted by Industry Leaders
”It’s the closest to having an extra member in our team. From alerts to CAPA follow-ups to structured reports, Qualifyze gives us everything we need while making the most out of our resources.”
“We chose to collaborate with Qualifyze, not only for their extensive audit database and fast delivery times but also for the precision and high quality of their reports.”
“Aenova is aware of this responsibility and therefore prefers to work with excellent partners, such as Qualifyze”
“Qualifyze helped us reduce costs and time thanks to their team of experienced auditors.”
“When Qualifyze audited us in 2021, we were impressed by the auditor’s qualification, precision, and profound knowledge. The audit also helped us a lot since it was comparable to an authority audit.”
“We have valued our experience working with Qualifyze on audit reports because of their reliable customer service, collaboration and thoroughness.”
“We chose Qualifyze because of their reliable customer service and the good quality of the audit reports at a fair price. Our requests were handled with flexibility and willingness to compromise.”







Check out our latest resources
Live Webinar “AI in CAPA Effectiveness: An Expert Discussion”
Where automation ends, and executive accountability begins AI is transforming pharma quality operations, but adopting it successfully is a leadership...

Whitepaper – The Case for AI Adoption Across Pharma Quality Operations

The Complete Guide to Supplier Qualification in 2026
